The Hypoxia Inducible Factor (HIF) is a transcription factor, which consists of an a and a β subunit. One of three α subunits dimerizes with one of two β subunits. The α subunits are sensitive to oxygen, whereas the β subunits are not.
In this video, the domain structure of an HIF1α subunit is shown. A domain in the center of the protein contains two critical proline residues, which are important for the stability of the protein. An arginine residue in the C-terminal transactivation domain regulates transcriptional activity. Under normoxic conditions, the tissue concentration of molecular oxygen is in the range of 10 to 30 µM. Under these conditions, a member of the prolyl hydroxylase domain family (PHD) hydroxylates one or both of the critical proline residues in the HIFα proteins. When a single or both of the critical proline residues have been hydroxylated, the von Hippel Lindau protein (pVHL), a tumor suppressor protein, binds to HIFα. The von Hippel Lindau protein associates with a ubiquitin ligase complex that causes the attachment of ubiquitin to HIFα. Polyubiquitinated HIFα is degraded by the 26S proteasome.
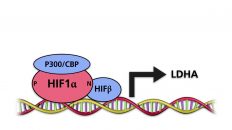
In addition to the proline residues, HIFα proteins contain a critical arginine residue in the C-terminal transactivation domain. The residue is hydroxylated by a dioxigenase designated factor inhibiting HIF1α (FIH1). The hydroxylated arginine residue inhibits the association of the p300 and CBP transcriptional co-activators with HIFα.
Under hypoxic conditions, the activity of PHD dioxigenases decreases. As a consequence, the proline residues of HIF are no longer hydroxylated, and the HIFa units are stabilized. A HIFα subunit associates with a HIFβ subunit. The heterodimer binds to a consensus sequence in the DNA. Since FIH1 no longer hydroxylates the arginine residue in the C-terminal transactivation domain of HIFα, the p300 and CBP co-activators are able to bind. The heterodimer activates the transcription of target genes.